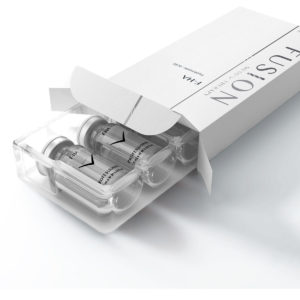
Fusion F-HAR Deep HA filler 25%
€ 89.00
Description: Only for use with the needle. Not for lippen!
Medium wrinkles and lips
Reticulated hyaluronic acid 25 mg/ml
The highest possible standards of effectiveness, durability, safety, and innovation thanks to the technological
expertise, the extensive know-how, the professionalism and the scientific rigor of the highly qualified staff.
F-HAR DEEP is dermal filler manufacture using biotechnologically manufactured cross-linked Hyaluronic
acid. F-HAR DEEP is a medical device with certification CE0373, it has been conceived, developed and
manufactured entirely in Italy. Highly tolerated and absolutely not painful at injection. It virtually does not
contain endotoxins as the hyaluronic acid is produced by bio-fermentation of a bacterial strain that is
non-pathogenic to human beings. F-HAR DEEP has a higher concentration of cross-linked Hyaluronic Acid
than other fillers on the market, its effects are immediately visible after the first application and it last up to
12 months.
INNOVATIVE MANUFACTURING PROCESS
The CE-certified manufacturing process implemented for F-HAR DEEP guarantees the sterility of the filler by
using a fully aseptic preparation method, thus avoiding the typical degradation process of conventional
heat sterilization. This provides more stability, preserves the active ingredient’s chemical and physical
characteristics, and guarantees long-lasting effects of the treatment over time.
HIGHLY SELECTED RAW MATERIALS
The Hyaluronic Acid selected is derived using a patented method based on the bio-fermentation of a
a bacterial strain that is non-pathogenic to human beings.
SAFETY GUARANTEE
Its high purity and very low concentrations of nucleic acids and proteins, as well as a bacterial endotoxin
level of < 0.2 EU/ml (markedly lower than the limits set by the European Pharmacopoeia for injectable
products) provide maximum reduction of hypersensitivity reactions.
Areas: Face.
Frequency of treatments: once every 8 to 12 months.
Description
Fusion Meso dermal fillers are produced using biotechnologically manufactured cross-
linked Hyaluronic acid. They are classified as medical device (CE0373) and exclusively
sold to plastic surgeons, dermatologists and aesthetic doctors. They are manufactured
entirely in Italy, highly tolerated and absolutely not painful at injection. They virtually don´t
contain endotoxins as the hyaluronic acid is produced by bio-fermentation of a bacterial
strain that is non-pathogenic to human beings. 2 concentrations are available and the
effects are immediately visible after the injection and it last up to 12 months.
INNOVATIVE MANUFACTURING PROCESS
The CE-certified manufacturing process implemented guarantees the sterility of the filler
by using a fully aseptic preparation method, thus avoiding the typical degradation process
of conventional heat sterilization. This provides more stability, preserves the active
ingredient’s chemical and physical characteristics, and guarantees long-lasting effects of
the treatment over time.
Its high purity and very low concentrations of nucleic acids and proteins, as well as a
bacterial endotoxin level of < 0.2 EU/ml (markedly lower than the limits set by the
European Pharmacopoeia for injectable products) provide maximum reduction of
hypersensitivity reactions.
Only logged in customers who have purchased this product may leave a review.






Reviews
There are no reviews yet.